Influence of natural decrepitation on the composition of melt inclusions in olivine: a study of melanocratic basalts from avacha volcano / kamchatka
Portnyagin M.V.1, Plechov P.Yu.2, Osipenko A.B.3
1 Vernadsky Institute of Geochemistry and analytical chemistry RAS, 117975, Moscow, Kosigin st.19.
2 Moscow State University, Geological department, 117234, Moscow, Leninskie gori.
3 Institute of Volcanology FEBRAS, 683006, Petropavlovsk-Kamchatsky, Piip boulevard, 9.
Key words: melt inclusions, experiment, decrepitation, island-arc magmatism, Kamchatka
Natural decrepitation of melt and fluid inclusions in minerals typically occurs in response of magma ascent at crystallisation and volcanic eruptions leading to significant difference between pressure inside inclusion and external pressure around host mineral [1,2]. In fact, decrepitation is a way of decompression of inclusion, and this may proceed by partial lost of initial content from melt inclusion. The degree of the leakage depends largely on compressibility of inclusion content [2]. Due to the weak compressibility, inclusions of magmatic melts undergo typically partial decrepitation when primary inclusion is visually preserved but accompanied by numerous secondary inclusions accommodating excess of volume. In this paper we address some geochemical consequences of partial decrepitation on the composition of melt inclusions illustrated by study of exotic olivine-clinopyroxene rich lavas from Avacha volcano named previously as "avachites" [3].
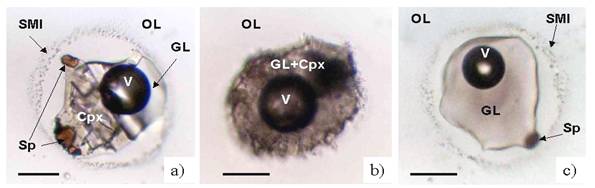
Avachites were described in first time in paper [3]. These rocks are basaltic in composition and contain 35-50 vol.% of large (up to 2 cm) phenocrysts of high magnesian olivine (Fo91-85) and clinopyroxene (Mg#92-87) in hyalo-ophitic high-alumina andesite-basaltic groundmass (Table 1). Abundant xenoliths of volcanic and intrusive rocks were also described [3]. The olivine phenocrysts contain numerous fluid and melt inclusions, crystal inclusions of clinopyroxene and chrome-rich spinel. The assemblage of inclusions is indicative of magmatic origin of the olivine crystals and suggests an origin of phenocrysts in avachites by cotectic crystallisation of olivine, clinopyroxene and spinel from high magnesium parental melt. At room temperature melt inclusions consist of dacitic glass, high calcium pyroxene (Table 1), spinel and fluid bubble. Melt inclusions in forsterite-rich olivines (Fo>87) are typically built by few well-shaped daughter crystals and are partially decrepitated as indicated by aureole of small secondary melt and fluid inclusion around the large primary inclusion (Fig.1a). Inclusions in relatively iron-rich olivines (Fo85-87) are finely crystallised and do not display signatures of decrepitation (Fig.1b).
In order to determine bulk
composition of recrystallised melt inclusions we have carried out a series of
heating experiments. During the runs single grains of olivines containing
inclusions were heated in He-atmosphere up to melting point of last daughter
clinopyroxene or some 10-30 degrees above and then rapidly (~200o/c)
quenched. A low-inertia heating stage with optical control [4] was
employed for the study. No complete homogenization was achieved in any of 15
experiments. Fluid bubble was always present in inclusions regardless the
temperature approached. Quenched inclusions contained glass, fluid bubble and
rare spinel crystal. If existed before experiment, an aureole of secondary
inclusions was also observed after quenching (Fig.1c). No correlation was found
between temperature of daughter clinopyroxene melting and composition of host
olivine.

Figure 1 Melt inclusions in olivine from avachites: a) inclusion in high magnesian olivine; b) inclusion in iron-rich olivine; ñ) melt inclusion in high magnesian olivine quenched at 1250 îÑ. Abbreviations: OL – host olivine, GL - glass, Cpx - clinopyroxene, Sp - spinel, V- fluid bubble, SMI – secondary melt inclusions. Scale bar is 30 mkm.
Representative compositions of partially homogenized melt inclusions are shown in Table 1. All analyzed inclusions have basaltic Ne-normative composition, whereas lavas of Avacha volcano are Hy-normative basalts approaching Qz-normative andesites and dacites. In comparison to host rock inclusions are characterized by low FeO and MgO, similar SiO2 and elevated CaO, Al2O3, Na2O, K2O, TiO2. Ratios of elements incompatible with olivine vary significantly. CaO/Al2O3 in melt inclusions tend to decrease with increasing iron content in host olivine. However, up to 2-fold variations of the ratio are observed for co-genetic melt inclusions within single olivine grain (e.g., analyses no 7-9 in Table 1). Na2O/K2O and K2O/TiO2 ratios in opposite remain almost constant for cogenetic inclusions but vary significantly for different grains. The variations of melt inclusions composition and their peculiarities when compared to host rocks can hardly be explained by single process and more likely are resulted from a complex of different processes, such as (1) diffusive re-equilibration of melt inclusions and host olivines, (2) mixing of compositionally variable parental melts and (3) partial decrepitation of melt inclusions.
Re-equilibration of melt inclusions and host olivines at low temperatures is a common feature of melt inclusions in minerals of island-arc rocks and readily explains systematically lower FeO content in inclusions compared to host rocks [5]. However, this process can not account for Ne-normative and high-Ca composition of majority of melt inclusions in avachites. This is because for olivine hosted melt inclusions, diffusive re-equilibration should decrease calcium in melt due to referable partition of calcium into olivine with decreasing of temperature [6]. Normative nepheline content should also decrease to compensate increasing of normative hyperstene proportion in the melt. The melt inclusions studied demonstrate reverse compositional trends. Clearly, this observation call into question a very genetic link between rocks of Avacha volcano and melts found in high magnesian olivines from avachites. Mass balance calculations and thermodynamic modeling of olivine and clinopyroxene crystallisation from compositions of melt inclusions further support this conclusion.
A straightforward explanation for the observed relationships between melt inclusions and rock chemistry is that olivine was crystallised from specific type of primitive Ne-normative high-Ca melts, which were not found so far among lavas erupted on the surface [7]. However, when applied to avachites, this explanation seems to contradict the data obtained. Host olivines of the melt inclusions have restricted CaO content (0.15-0.20 wt.%), which does not correlate with composition of the trapped melts. In more general view, high-Ca melts could not be equilibrated with typical mantle sources [7], and assimilation of common clinopyroxenites by ascending magmas was shown not to produce Ne-normative melts [8]. Thus, if large volumes of silica undersaturated high-Ca melts really existed in nature, one should imply origin of these magmas by involvement of "exotic" low-Si pyroxene component of unknown provenance in mantle melting or assimilation process. Fractionation from parental silica-undersaturated melts to typical basalts and andesites seem to be not simple as well and should involve amphibole as fractionating phase [7] or silica-rich contaminant. Clearly, the idea of primary nature of silica undersaturated high-Ca melts found in melt inclusions meets a large number of unknowns.
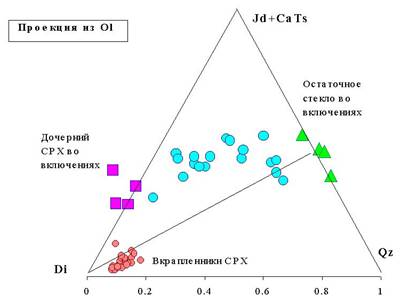 Fig.2
The compositions of
partially homogenized melt inclusions, daughter phases in recrystallised melt
inclusions and clinopyroxene phenocrysts from avachites plotted in CIPW
normative tetrahedron, projected from olivine onto base jadeite plus calcium
tschermak's molecule (Jd CaTs) - quartz (Qz) - diopside (Di) [9].
Fig.2
The compositions of
partially homogenized melt inclusions, daughter phases in recrystallised melt
inclusions and clinopyroxene phenocrysts from avachites plotted in CIPW
normative tetrahedron, projected from olivine onto base jadeite plus calcium
tschermak's molecule (Jd CaTs) - quartz (Qz) - diopside (Di) [9].
Compositional variability of primitive melts is proven to exist in nature. However, alternative and intuitively more realistic explanation for the origin of silica undersaturated high-Ca melts from this study is influence of partial decrepitation on the composition of melt inclusions as microscopic observations provide strong evidence for the suggestion (Fig.1). Let us assume that decrepitation took place at the moment when large daughter clinopyroxene in inclusions was crystallised already. Then, predominant leakage of residual melt from inclusion will shift bulk composition of the inclusion to enrichment in component of daughter clinopyroxene composition. In general case, shift of bulk inclusion composition will be controlled by the chemistry of daughter phases existed inside inclusion at the moment of decrepitation. The data of this work appear to support this assumption. The geochemical effect of decrepitation is demonstrated in Fig.2, where normative compositions of reheated melt inclusions fall along trend between compositions of major daughter phases in inclusions. The composition of daughter clinopyroxene in melt inclusions is highly unusual for basaltic magmas and differs significantly from composition of clinopyroxene phenocrysts in the same rocks. The most important peculiarities are low SiO2 and high Al2O3 and TiO2 contents. These features reflect high proportion of Ñà- è Ca-Ti- tschermak's molecule in pyroxene composition which amounts ~20 mol.% whereas majority of magmatic pyroxenes contain around 2-5 mol.% tschermak's molecule. These compositional peculiarities of daughter pyroxene seem to be responsible for the shift of bulk inclusion chemistry to silica undersaturated high-Ca field.
Based on the data obtained one may conclude that partial decrepitation of melt inclusions influenced strongly the composition of melt inclusions in the most magnesian olivines which probably crystallised at highest pressure. An origin of silica-undersaturated high-Ca melts is explained by excess of daughter low-Si high-Al clinopyroxene in melt inclusions, which were remelted in high-temperature experiments. Pyroxene of similar composition is described in melt inclusions from many olivine-bearing primitive samples of different provenance [10, unpublished data]. Thus, one may expect that geochemical effect of decrepitation will be also similar in many cases especially when clinopyroxene is an early liquidus phase of melts. Taking into attention wide spread of naturally decrepitated magmatic inclusions [1,2], our results emphasize one more time an importance of detailed microscopic study of magmatic inclusions in order to avoid erroneous results.
This work is supported by Russian Foundation for Basis Research (Grant ¹ 00-05-64384).
References cited:
[1] Roedder E. // Miner. Soc. Amer. Michigan: Book Crafters Inc. 1984. 644 pp.
[2] Tait S. // Am. Miner. 1992. V. 77. pp. 146-155.
[3] Kutiev F.Sh. et al. // Dokladi Akademii Nauk. 1980. V. 255. No 5. pp. 1240-1243.
[4] Sobolev A.V., Slutsky A.B. // Geologiya and Geofisika. 1984. No12. pp. 97-110.
[5] Danyushevsky L.V. et al. // Contrib. Miner. Petrol. 2000. V.138. p.68-83.
[6] Libourel G. // Contrib. Miner.Petrol. 1999. V.136. p.63-80.
[7] Schiano P. et al. // Abstr. 9th Goldschmidt Conf. August 22-27. 1999. CD volume.
[8] Hirschmann M.M., Schiano P. // Abstr. 9th Goldschmidt Conf. August 22-27. 1999. CD volume.
[9] Falloon T.J.,Green D.H. // Mineralogy and Petrology. 1987. V. 37. ¹ 3/4. P. 181-219.
[10] Della-Pasqua F.N. et al. // Mineralogy and Petrology. 1995. V. 53. p. 1-26.
Table 1. Major elements content in samples studied (wt.%)
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|
Sample |
WR |
GM |
Gl-i |
Cpx-i |
Av1 |
Av3 |
Av4-a |
Av4-b |
Av4-c |
Av10a |
Av14 |
|
SiO2 |
50.6 |
54.5 |
64.8 |
44.3 |
48.6 |
47.8 |
49.5 |
49.7 |
50.2 |
47.8 |
50.5 |
|
TiO2 |
0.72 |
0.57 |
0.47 |
1.48 |
0.73 |
1.18 |
1.22 |
1.14 |
1.02 |
0.74 |
1.33 |
|
Al2O3 |
10.3 |
18.6 |
21.1 |
13.5 |
13.6 |
13.7 |
14.3 |
16.6 |
12.0 |
18.2 |
18.7 |
|
FeO |
8.46 |
6.20 |
0.72 |
6.49 |
6.70 |
5.57 |
6.00 |
5.71 |
6.33 |
6.62 |
5.49 |
|
MnO |
0.14 |
0.14 |
0.05 |
0.09 |
0.10 |
0.13 |
0.10 |
0.11 |
0.36 |
0.23 |
0.28 |
|
MgO |
16.2 |
4.90 |
0.40 |
10.9 |
11.1 |
13.2 |
10.4 |
9.90 |
10.8 |
8.98 |
8.67 |
|
CaO |
11.5 |
10.3 |
2.25 |
22.6 |
15.3 |
14.5 |
14.5 |
12.5 |
15.4 |
13.4 |
9.24 |
|
Na2O |
1.77 |
3.65 |
8.37 |
0.67 |
3.20 |
3.02 |
3.15 |
3.31 |
3.03 |
3.56 |
4.79 |
|
K2O |
0.30 |
0.58 |
1.56 |
- |
0.43 |
0.81 |
0.71 |
0.82 |
0.73 |
0.45 |
0.88 |
|
Cl |
- |
0.07 |
0.29 |
- |
0.12 |
0.08 |
0.12 |
0.19 |
0.17 |
0.10 |
0.18 |
|
Fo, mol% |
- |
|
- |
- |
90.7 |
89.4 |
88.6 |
88.6 |
88.6 |
86.0 |
85.0 |
|
CaO/Al2O3 |
1.12 |
0.55 |
0.11 |
1.68 |
1.12 |
1.06 |
1.01 |
0.75 |
1.28 |
0.73 |
0.49 |
1 - avachite bulk composition [3];
2- groundmass composition of avachite (average of 5 broad beam EMP analyses) ;
3-4 - average composition of daughter phases in 4 recrystallised melt inclusions
in olivine Fo87-89 (3 - glass, 4 - clinopyroxene);
5-11 - partially homogenized melt inclusions. Fo - host olivine Mg-number. All
compositions are normalized to 100 wt.%.