

|
Electronic version. Complete one was printed as V.A.Maltsev. Stalactites with "internal" and "external" feeding, Proc. Univ. Bristol Spel. Soc., 1998, 21(2), 149-158.Turn to shortened Russian version.
STALACTITES WITH "INTERNAL" AND "EXTERNAL" FEEDING. by Vladimir A. Maltsev VNIIGEOSYSTEM INSTITUTE, Leninsky prosp. 61/1-57, Moscow 117333, Russia
ABSTRACT. The conventional stalactites classification via their feeding mechanisms seems to be wrong. The central channel of the carbonate stalactites, with rare exceptions, is shown to have no special role in the stalactite feeding, thus appearing as a consequence of the growth mechanism, and not as a cause. In the case of conical stalactites even partial feeding through the channel seems to be prohibited. Non-carbonate stalactites (except those growing from melts), have a structure and texture, corresponding, to that of tuflactites, corlactites, or crystlactites not to real stalactites. Of these, a significant feeding role for the central channel appears only for the tubular tuflactites. INTRODUCTION. It is normally considered that stalactites have internal feeding (tubular or soda-straw), and external or mixed feeding (cone stalactites) (Hill and Forti, 1997; Maximovich, 1965; Stepanov, 1971, More, 1962). However, most visitors to a karst cave with actively growing stalactites will probably have noticed the following: If a cone stalactite, having a central channel, had been broken several years ago, and new growth had started at its end, the new stalactite nearly always appears at the lowest point of the broken surface, always has a channel, and this channel almost never continues the old channel (photo 1). In addition, if the broken object consists of a pair of a soda-straw and a drapery and the lowest point of the break is within the drapery, the new growth starts at this point, where any feeding channels are impossible, the growing object is a soda-straw (a stalactite with "internal" feeding), and there is no communication between the channels of old and new soda-straws. There is other evidence that the concept of stalactites central feeding is not trivial, this will be considered below. This paper will only consider in detail the case of carbonate stalactites, outlining other cases, which have different crystallization physics and chemistry and thus produce stalactites with very different structure, only in general.
SODA-STRAWS Soda-straws are a good object to begin the study as they are the "clearest" type of stalactite with a feeding channel and their monocrystalline structure is quite simple. Other types of tubular stalactites, grainy tubular stalactites or tubular tuflactites (Stepanov, 1997, Maltsev, 1997), principally differ from soda-straw in their structure, texture, crystallization mechanisms and feeding mechanisms and will be considered below. 
Soda-straws have been proved to appear within environments with low values of feeding speed (Maximovich, 1965; Stepanov, 1971) and low values of oversaturation of the solution by CO2. In many cases, soda-straws grow in large quantities, separately from other types of stalactites, and are spread uniformly over the roof. This uniformity, locally controlled not by the geometry of feeding fractures, but by their growth at points favorable for dripping (as in the example of the broken stalactite), can be taken to statistically disprove the idea of their feeding via their channels. Also, the fact, that some of them are followed by draperies, having their volumes greater than soda-straws, directly shows presence of external (surface) feeding. This was confirmed by examination of the substrate under soda-straws; the author studied it in dozens of samples. Not one sample was found which showed a feeding channel, even a capillary one. Probably/ everybody seen soda-straws, growing upon broken stalactites (photo 1), never continuing the original channel, but growing on the lowest point of the break. Now let us consider the crystallization physics in the dripping point. In "usual" cave conditions the only mechanism for creating the oversaturation of a solution by Calcium is by CO2 loss during bicarbonate decomposition (Sokolov, 1962). It is also known that mechanical agitation of the solution is frequently the reason for bicarbonate decomposition occurring in balanced solutions. This is the reason, for bicarbonate decomposition occurring in balanced solutions. This is just the same, because mechanical agitation simply produces local pressure irregularities, and solubility function of CO2 gas is very steep, thus providing major saturation irregularities in localities of minor pressure irregularities (Stepanov 1971). This is the reason, for example, for the growth of gours caused by turbulence on the overflows. The dripping frequency, typical for soda-straws, about 1-0.001 drops/second, is too low to cause significant turbulence. Thus, there are only two possibilities left for the crystallization: 1) an initially oversaturated solution. In this case, stalactite growth will take only part of the CaCO3, the falling drops would be saturated or oversaturated and stalagmites will definitely appear because of the explosive mechanics-caused degasation, and these stalagmites would be most likely definitely travertine ones, not crystalline. The paragenetic pairs soda-straw-stalagmite are known, but they are rare, and the stalagmites are always crystalline. This means, that the drops land being slightly undersaturated, subsequently; thus the initial solution is approximately balanced; 2) local degasation, occurring at the moment of the drop's disconnection, and caused by mechanical reasons - the meniscuses gap. 
It is evident, that it is only necessary to consider the last mechanism. For the same reason, it is evident that the main mechanical agitation is at the gap perimeter, where between situations of positive and negative meniscus the force of surface tension momentarily breaks its direction almost to opposite. This means, that the crystallization will be favorable on the meniscus perimeter, automatically causing the tubular structure of the growing stalactite. Let us examine the additional evidence of this mechanism, the structure of the soda-straw's growth zone (photo 2). The immediate crystallization on the meniscus perimeter results not in the main crystal, but in a set of skeleton crystals and dendrites. This is a well-known feature of growth during mechanical degasation (Shafranovskiy, 1961, Stepanov, 1971, 1997). At a distance of 0.5-3 mm from the end of the soda-straw, these skeleton crystals and dendrites are completely replaced with the main crystal, building the soda-straw body (Maltsev, p106 in Hill and Forti, 1997). This means re-crystallization, also with some peculiarities of the environment. If the solution is oversaturated, some of the skeleton crystals must have overgrowths, conserving their structure. Complete re-crystallization is possible only in the balanced solution (and is necessary - dendrites and skeleton crystals are poorly balanced formations). So, this structure completely shows once more the crystallization process anatomy: crystallization at the moments of the drop's disconnection, and re-crystallization during the periods of its static hanging. It should be noted that the mechanism discussed does not depend on the feeding mechanism. If dripping is rare, and the solution is balanced then the soda-straw must appear with both internal and external feeding, as a result of the crystallization physics. With this, external feeding is evidently preferable for mechanical reasons; the dripping points are controlled mostly by the roof geometry, not the geometry of feeding fractures. The absence of crystallization on the outside surface of the soda-straw is explained by the fact that the solution is balanced and that as the feeding is slow enough, the flow is laminar. So, Maximovich was right, differences between the various stalactites do not depend on the feeding structure but only on the feeding speed, and the saturation grade. There are many observations which show the presence of internal feeding for the soda-straws (Hill and Forti, 1997), and the absence of external feeding. This does not contradict the above. These observations are correct, but apply only to long soda-straws, which have been the main object of past observations. The walls of a soda-straw are about 0.5-1.5 mm thick and the carbonates have perfect cleavage. This results in a soda-straw having fractures in its wall. The water column inside the soda-straw creates a strong negative pressure in its upper part, proportional to its length, and which oscillating during dripping. For these reasons water is sucked in through these fractures and for straws of a certain length (10-20 cm) the surface flow can disappear completely. Further evidence for this is the case of long soda-straws having overgrowths on their upper parts, thus demonstrating surface flow, but having no surface flow in their lower parts. So, both the existence of the channel and feeding through it appear not to be reasons for the growth of the soda-straw, but the opposite, consequences of its growth. 
The author knows of only one exception when the feeding of a soda-straw appears to be originally internal. These are soda-straws growing from helictites (photo 3). This exception still supports the common rule, this is a special case where the favorable dripping point is the feeding channel opening. Thus it can be seen that soda-straws can be fed either internally or, more frequently, externally and yet the same structure will result.
CONE STALACTITES. Two theories have been put forward to explain the structure and genesis of cone stalactites (this discussion is limited to varieties which have an internal channel). According to the first (Hill and Forti 1986), they are soda-straws overgrown by a surface crust at a later stage. According to the second one (Maximovich, 1965; Stepanov, 1971, 1997), the central tube is syngenetic to the crust, and they grow simultaneously. The author believes that the second theory is the right one. Maximovich has statistically proved that soda-straws grow at much lower feeding speeds than cone stalactites. Stepanov showed that crystallization in caves is cyclic and within each cycle the rate of feeding decreases. Thus, in a given cycle soda-straws will appear after cone stalactites, not before. Between cycles there will be a dissolution period, that would leave traces between a soda-straw and its overlying crust and these have not been found. On the contrary, inductional surfaces of simultaneous growth can be found in each cone stalactite. So, let us discuss the simultaneous growth of the central tube and the crust. To begin with, to simplify the considerations, we will accept the hypothesis that the solutions inside and outside the stalactite are originally the same. Variations, of course, exist, but are relatively rare. Let us now examine what happens with such "mixed" feeding. The solution coming along the central channel, can only lose CO2 at its very end. This is not just a theoretical consideration, otherwise crystallization inside the channel would be seen. The solution on the surface, however, can lose CO2 at any time and so while it is moving it will deposit the crust. At the end of the stalactite there will be mixing of the two solutions. the internal one is balanced, in CO2, to the feeding fracture conditions, the surface one to the surrounding air conditions. Both are saturated in Ca relative to CO2, the internal one oversaturated in CO2, the surface one about to be balanced in CO2. As is well known, the Ca(CO2) saturation function is convex everywhere. (Palmer, 1995 has a good set of graphs showing this.) The mixture of two Ca-saturated solutions is always represented by some point on a chord connecting points which represent source solutions. As all the chords lie completely below the function, every such mixture is undersaturated in Ca. This causes the well-known effect of "mixing corrosion", that is considered to be one of the main mechanisms for the karstification of limestone. Of course, this mechanism will work only while there is no time for or possibility of additional CO2 loss. According to Maximovich (1965), dripping rates from cone stalactites are high, much greater than 1 drop/sec. This means that the mixture will have less than 1 second for additional degasation, evidently not enough. In the case of mixed feeding we would therefore see corrosion instead of crystallization. So, we reach a nonsensical position: No mixed feeding is possible until feeding amounts along two "branches" are proportional. One of the flows must be several orders greater than the other and that may then simply be ignored, even if exists. A comparison between the material deposited by central tube and on the crust shows that the surface flow is certainly significant, so the internal feeding must be the insignificant "branch". Finally it must be noted, that nothing changes if the hypothesis of a common origin for both solutions is ignored, the mixing effects would still be present. 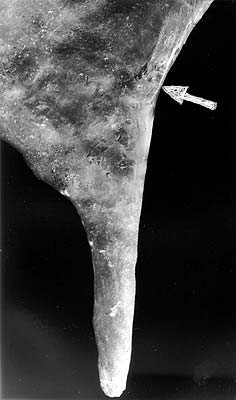
See Figure 4 for an illustration of the above. This shows a formation intermediate between a cone stalactite, and a paragenetic pair of drapery and soda-straw (the only difference between them is in plain versus linear surface feeding). The curvity of the monocrystalline tube (soda-straw) in the upper half is seen well and shows clearly that the tube and the drapery are syngenetic and that the tube had external feeding from the drapery at least on its first stage. In the lower part the tube is straight. This sudden straightness, together with the clearness (without any overgrowths) of a large part of the soda-straw's surface, show a sudden feeding reconstruction, that the sucking of the solution into the channel is not sequential, but mostly goes through some accidental hole. This hole may be seen in the upper left corner of the aggregate. What's the origin of the curve? If feeding is surface and uni-directional, then the geometry of each next drop is distorted by surface tension of the flow, so the dripping point moves against the feeding source until length of the stalactite becomes enough to eliminate this force. Second moment. The soda-straw part is curved not very smooth because another compromiss is needed - between this force and crystallisation forces, and this leads to "polyline" shape. And the hole is reffered just to illustrate, that on thin-wall soda-straws sucking in is interactive, it can enlarge one of the fracture holes.
NON-CARBONATE STALACTITES. In the case of non-carbonate stalactites, the solution oversaturation may come for two reasons, neither connected with mechanical agitation. These are: evaporation while the drop hangs static drop and pressure/temperature condition jumps between the feeding channel and the cave. 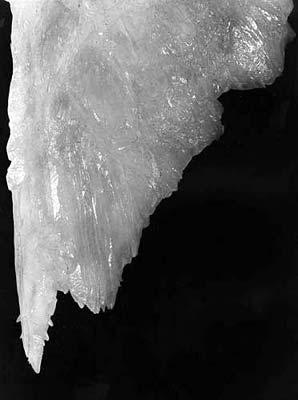
The first variant is analogous with the carbonate case: the importance of evaporation grows with the decrease in dripping frequency. So, a carbonate stalactite with very slow dripping will receive some morphological, structural, and textural features from evaporation-controlled formations. As shown by Stepanov (1971), with slow feeding and low humidity, allowing evaporation, stalactites are transformed into corlactites or crystlactites. The two last are the formations, significantly controlled by capillarity, intermediate between stalactites and corallites (or crystallictites). Corlactites and crystallictites have the following features: their gravitational control is weakened; capillary film kinetics control is enforced; crystallographic control is enforced; their surface is often faced; a central channel is optional and, if it exists, has irregular geometry, not controlled by the meniscus. 
The author's studies in caves where there is growth of sulfates (in all these caves oversaturation is caused by evaporation), shown that the stalactites always have a structure and texture which is more complex than in the carbonate case. In the most simple environments they are similar to carbonate crystlactites (photo 5), in more complex they convert into chandeliers (photo 6). Tubular cases with feeding channels are also known, but aren't soda straws, like a halite stalactite on photo 7. Corlactites are not found because too high oversaturation is needed for sulfate crystals to become split. A gypsum "stalactite" (really crystlactite) has an internal channel in only about half of the cases (having irregular geometry), has a faced surface, as a rule is not vertical and is often branched. In the localities where the dripping is frequent enough to suppress these features, no stalactites are found, evaporation is too slow for growth when there is fast feeding. The second variant also has some analogies in the carbonate case. These are spring swells, shields, other formations, generated from rapid degasation on the condition barriers. They appear in the points of feeding fractures opening when there is such a barrier between the cave and the fracture. They may be vertical, and may have a channel. Their structure is grainy, up to tufa, no monocrystalline tubes appear, gravitational control is weakened. The channel has irregular geometry. The most "stalactite-like" formations of this kind are tufa tubular stalactites, usually growing in mines within ore deposits (the author met them in the Khaidarkan mines), where conditions in feeding fractures and in the growth space (mines) are strongly disbalanced. They are also characterized by weakened gravitational control, absence of monocrystalline tubes and irregular channel geometry. In Stepanov's classification, they are not stalactites, but tuflactites, tubular variety. All the tubular stalactite-like non-carbonate aggregates, which have their genesis driven by pressure/temperature conditions, both observed by author and recognizably mentioned in literature (i.e. Plavshudin 1973), have all the features of tubular tuflactites, and never show anything similar to soda-straws.
STALACTITES CRYSTALLIZING FROM MELTS. Let us study the example of crystallization from melts, usually ice or sulfur. Surprisingly, this environment produces aggregates which have the maximum similarities to the usual carbonate case. Here can be found real stalactites, including monocrystalline soda-straws. The reason for this is simple: When a melt drop hangs in the cold air, its outside layer will be supercooled. Mechanical agitations on the meniscus perimeter during the drop's disconnection (sharp pressure fall) cause this supercooled melt to crystallize in an explosive manner. The physics is almost the same as in the carbonate case, explosive crystallization at the moment of the drop's disconnection, resulting in skeleton crystals and dendrites, and subsequent re-crystallization of these dendrites into a monocrystalline tube. And within rather intensive dripping - no real possibility of supercooling. But the strong local pressure falls raise freezing temperature in the connection zone (opposit effect to what is used in the idea of skating). The ice case is even more illustrative than the carbonate one. Icicles, growing outside caves and more than 1 day old cannot have an active feeding channel, in the night the melt inside will be frozen, and there is no mechanism which will open the channel again. the following day the icicle continues growing with undeniable surface feeding and again grows as a soda-straw. Yet again, the channel which is always present (Makkonen, 1988) appears to be not a cause of growth, but a consequence.
CONCLUSIONS The classification of stalactites by their feeding mechanisms does not correspond to considerations of their structure and texture and in the most common cases is simply wrong. Real stalactites, appearing as a result of the degasation of bicarbonate solutions, or the freezing of melts, have a central channel not by reason of their tubularity, but as a consequence of their crystallization physics. The occasionally appearence of internal feeding for these stalactites is a secondary effect. Tubular tuflactites are mostly characteristic for crystallization, driven by pressure/temperature condition barriers. These aggregates, significantly differing from stalactites in their structure and texture, may form from any sufficiently soluble minerals. These are the only stalactite-like aggregates with a real feeding role for the central channel. Corlactites and crystlactites, appearing within prevailing role of evaporation, also may be formed from any sufficiently soluble minerals. They also have strong differences from stalactites in their structure and texture. A central channel is optional, but if it exists it is a real feeding channel. Its existence or absence has an influence upon the aggregate's texture, but not structure.
REFERENCES C.Hill, P.Forti. Cave minerals of the world// NSS, 1986, 238 p. C.Hill, P.Forti. Cave minerals of the world. Second edition// NSS, 1997, 463 p. B.A.Makkonen., 1988. A model of icicle growth//Journal of Glaciology, vol.34, No. 116, pp. 64-70. V.A.Maltsev., 1997. Stalactites, crystlactites, corlactites, crystlactites: 4 types of "stalactite-like" formations, generated from crystallization environments with different physical properties //Transactions of 12-th Int. Congr. of Speleology, p.267-270. G.A.Maximovich., 1965. The genetic row of cave flowstone formatoins (carbonate speleolithogenesis) (in Russian) //Peshchsery, No.5(6). A.N.Palmer., 1995. Geochemical models for the origin of macroscopic solution porosity in carbonate rocks// In book: D.A.Budd, P.M.Harris, A.Saller (eds). Unconformities in carbonate strata: their recognition and the significance of associated porosity. American Association of Petroleum Geologists, Memoir 63, p.77-101. V.G.Plavshudin., 1973. Cryptomelane stalactites from the Nikopolskoye deposit (in Russian)//Zapiski VMO, vol. 102, no. 2, pp.213-215. I.I.Shafranovskiy., 1961. Crystals of minerals. Curved-faced, skeletal, granular forms// Moscow, Gosgeoltechizdat, 180 p. D.S.Sokolov., 1962. Main conditions of karst development (in Russian)// Moscow. V.I.Stepanov., 1971. Crystallisation processes periodity in karst caves (in Russian)// Trudy mineralogicheskogo muzeja imeny Fersmana. Moscow, No.20., p.161-171 V.I. Stepanov., 1997 Notes on mineral growth from the archive of V.I. Stepanov. Proc. Univ. Bristol Spelaeol Soc., 1997, 21(1), 25-42

|